As the world grapples with the pressing challenges of climate change and energy security, the search for clean and sustainable energy solutions has intensified. Among the promising technologies that have emerged, fuel cells stand out as a beacon of hope, offering a transformative pathway towards a decarbonized society. At the core of fuel cells lies the enigmatic field of electrocatalysis, a discipline that holds the key to unlocking the full potential of this transformative technology.
4.6 out of 5
| Language | : | English |
| File size | : | 24095 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 1285 pages |
| X-Ray for textbooks | : | Enabled |
What is Electrocatalysis?
Electrocatalysis is the study of how the rate of an electrochemical reaction can be increased by the presence of a catalyst. In the context of fuel cells, electrocatalysts play a crucial role in facilitating the key electrochemical reactions that convert chemical energy into electrical energy. The most common electrocatalysts used in fuel cells are based on platinum (Pt),a precious metal known for its exceptional catalytic activity. However, the high cost and scarcity of Pt have spurred the development of non-Pt catalysts, such as transition metal oxides and carbon-based materials, as more affordable and sustainable alternatives.
The Role of Electrocatalysis in Fuel Cells
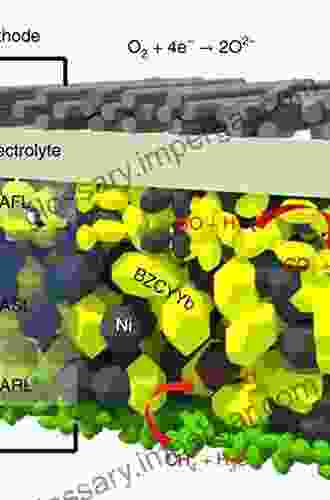
Fuel cells are electrochemical devices that convert chemical energy from a fuel, typically hydrogen, into electrical energy. The heart of a fuel cell consists of two electrodes, the cathode and the anode, separated by a proton-conducting membrane. Hydrogen is fed to the anode, where it undergoes an electrochemical reaction catalyzed by a hydrogen oxidation reaction (HOR) catalyst. This reaction generates protons, which pass through the membrane, and electrons, which flow through an external circuit, producing electrical energy.
At the cathode, oxygen from the air is reduced in an electrochemical reaction catalyzed by an oxygen reduction reaction (ORR) catalyst. This reaction consumes the electrons that pass through the circuit and combines with the protons that have passed through the membrane to form water.
The efficiency and performance of fuel cells are critically dependent on the activity and stability of the electrocatalysts used. Highly active electrocatalysts enable faster reaction rates and higher power densities, while stable electrocatalysts ensure long-term durability and reliability.
Electrocatalysis in Alkaline Fuel Cells
Alkaline fuel cells (AFCs) have gained increasing attention as an alternative to proton exchange membrane fuel cells (PEMFCs). AFCs operate at higher temperatures and use a liquid alkaline electrolyte instead of a proton-conducting membrane. This unique design offers several advantages, including lower cost, higher tolerance to impurities, and the ability to use non-noble metal catalysts.
Electrocatalysis играет решающую роль в AFC. Разработка высокоактивных и стабильных не-Pt катализаторов для ORR является критически важной для повышения производительности и долговечности АФЦ и снижения их стоимости.
Electrocatalysis in Direct Methanol Fuel Cells
Direct methanol fuel cells (DMFCs) offer a promising approach to power portable devices and vehicles due to their high energy density and ease of transportation and storage. Unlike hydrogen fuel cells, DMFCs use methanol as a fuel, which is oxidized directly at the anode.
The electrocatalysis of methanol oxidation is a complex process that requires highly active and selective catalysts. Pt-based catalysts are typically used for this reaction, but research is ongoing to develop more affordable and efficient non-Pt alternatives.
Challenges and Future Prospects
Despite the tremendous potential of electrocatalysis in fuel cells, several challenges remain to be addressed:
- The high cost of Pt-based catalysts
- The limited activity and stability of non-Pt catalysts
- The degradation of electrocatalysts over time
- The need for improved understanding of the complex reaction mechanisms involved
Future research efforts will focus on addressing these challenges through the development of novel electrocatalysts with improved activity, stability, and cost-effectiveness. Advanced characterization techniques and theoretical modeling will play a crucial role in unraveling the intricate mechanisms of electrocatalysis and guiding the design of new materials.
Electrocatalysis in fuel cells is a rapidly evolving field that holds immense promise for the development of clean and sustainable energy technologies. By unlocking the transformative power of electrocatalysis, we can harness the potential of fuel cells to decarbonize our energy systems, mitigate climate change, and create a more sustainable future for generations to come.